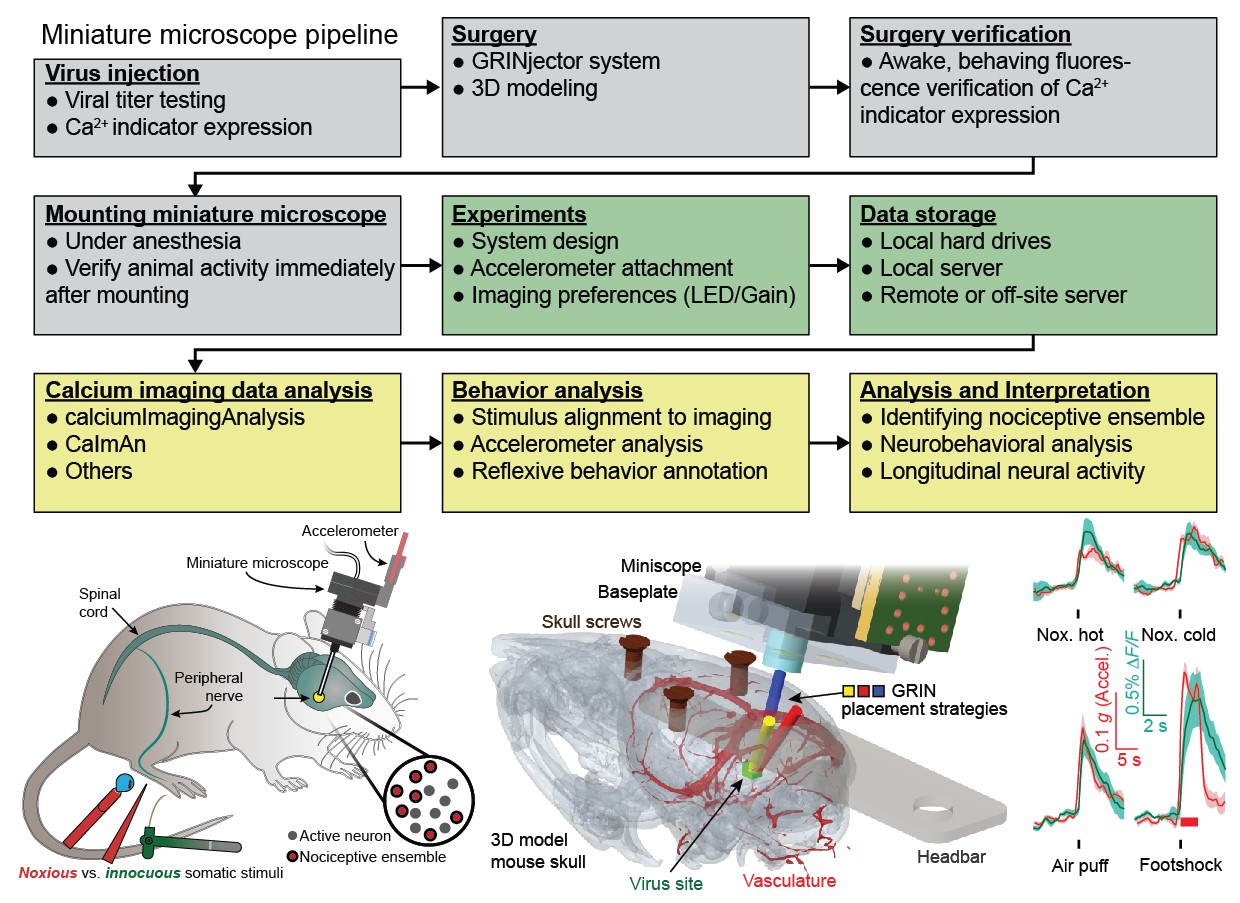
Our book chapter detailing the entire pipeline from surgeries to analysis for calcium imaging of pain-related neural activity has been published.
This can prove a useful reference for those new to calcium imaging (esp. in freely moving animals) or researchers who want to see how others perform certain experimental and analytical steps along with the rationale. Please reach out if you have any specific questions, happy to help!
During my doctoral work, in Prof. Mark Schnitzer's lab at Stanford, I advanced and developed a number of experimental and computational techniques to enable improved analysis of neural activity in freely moving animals. To facilitate sharing of these techniques and procedures, Gregory Corder (Asst. Prof at UPenn, check out his lab's awesome work: Corder Lab) and I wrote a book chapter that goes over the various steps needed to conduct successful calcium imaging experiments, from surgeries to analysis of imaging data and neural responses. This should help both existing and new researcher see what others are doing and the rationale behind some of those decisions.
The book chapter webpage and PDF are located below. Please reach out if you have any questions about calcium imaging experiments or analysis, happy to help!
- Ahanonu B., Corder G. (2022) Recording Pain-Related Brain Activity in Behaving Animals Using Calcium Imaging and Miniature Microscopes. In: Seal R.P. (eds) Contemporary Approaches to the Study of Pain. Neuromethods, vol 178. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2039-7_13
- Link to PDF of the book chapter.
Abstract
Pain is a multifaceted percept formed by information processing in the brain of ascending signals from the periphery and spinal cord. Numerous studies in humans and animals, using technologies such as fMRI, have demonstrated that noxious stimuli activate a distributed network consisting of multiple brain regions. These human and preclinical studies suggest that the nervous system relays nociceptive information through a vast network of high-order cognitive, motivational, and motor-planning brain regions to generate the perception of pain and resulting nocifensive behavior. While these previous studies have improved our understanding of brain network function in pain, they present limitations due to low-resolution, static snapshots of neural activity, or a difficulty tracking the same cells longitudinally across extended periods of time ranging from weeks to months. Here we present a protocol that uses recent advances in in vivo microscopy and computational techniques to address these questions. Miniaturized fluorescence microscopes (miniscopes) using microendoscopy allow for imaging of intracellular Ca2+ transients, which function as a proxy for neural activity. This innovative technology permits high-resolution imaging of large neuronal populations (up to 1000+ neurons in a single animal) located in deep brain regions of freely behaving mice over a time scale of months. This technology puts researchers in a position to answer many fundamental questions regarding the coding principles of nociceptive information and to identify pain-specific neural pathways in the brain. Furthermore, it is now possible to determine how brain neuronal networks evolve their activity dynamics over several months, before, during, and after chronic pain has developed while also understanding how existing and novel analgesics restore both behavior and neural activity to alleviate pain.
Links for a number of resources related to the book chapter.
- https://github.com/bahanonu/ciatah - My CIAtah calcium imaging analysis software, supports multiple methods/algorithms for certain steps of the pipeline.
- https://github.com/bahanonu/GRINjector - Tool to inject GRIN lens probes into animal brains or other tissue.
- https://github.com/bahanonu/imaging_tools - Table of imaging analysis algorithms and software, with a focus on calcium imaging.
 stanford
stanford linkden
linkden github
github goodreads
goodreads medium
medium twitter
twitter